Apatite II has no fluorine substitution in the hydroxyl position,
Apatite II has a high degree of carbonate substitution,
Apatite II is generally amorphous with random nanocrystals of apatite,
Apatite II has few trace metals, and
Apatite II has a high degree of microporosity and surface area.
The ultimate driving force for the robust performance of reactive phosphate with respect to metals is the extreme stability of these metal-phosphate phases, some of which are listed below. These metal phosphates are 20 to 70 orders of magnitude more insoluble than quartz. Combined with this stability, the rapid kinetics of the metal-phosphate precipitation ensures immobilization of the metals in the environment of most possible transport mechanisms.
Table 2.1 - Stability of Apatite Minerals
| Mineral Phase | Solubility Product (log Ksp) | Mineral Phase | Solubility Product (log Ksp) |
| Pb5(PO4)3(OH,Cl) | -76.5 | Am(PO4) | -24.8 |
| Ca(UO2)2(PO4)2 � 10H2O | -49.0 | Pu(PO4) | -24.4 |
| Sr5(PO4)3(OH) | -51.3 | UO2HPO4 | -10.7 |
| Zn3(PO4)2 | -35.3 | Quartz (SiO2) | -4.0 |
| Cd3(PO4)2 | -32.6 | Salt (NaCl) | 0.0 |
2.1.1 Typical Emplacement Methods
Site specific questions regarding PIMS involve how to emplace the Apatite II, or how to bring the soluble metal into contact with the Apatite II. The following are typical emplacement methods.
Soil mixing is a method in which Apatite II is mixed with the contaminated soil so that any soluble metal is immediately immobilized and metal dissolved from lead particulates in the future is subsequently immobilized as it begin to migrate. The amount of Apatite II added depends on the metal inventory and other site parameters. This stabilization method treats mobile soluble metal as it begins to migrate, not the particulate metal as it exists in the soil. This stabilization method does not remove metal from the soil but stabilizes the metal against future leaching.
A permeable reactive barrier, emplaced in a trench downgradient of contaminated groundwater or contaminant source, is a method that treats the soluble metal in the water as it moves through the barrier and immobilizes the metal as an insoluble metal-phosphate phase, such as lead-pyromorphite. Size of the barrier depends on the expected metal inventory, geometry of the plume, and desired treatment period. This method removes soluble metal from the groundwater. Apatite II can be used in combination with other reactive media, such as zeolites, iron filings, or a biobarrier for treating a mixed waste plume, either mixed into the same barrier or into a sequential multi-barrier configuration.
A liner or other disposal components composed of Apatite II surrounding or underlying a site is a method in which the Apatite II treats leachate migrating out of a contaminated waste disposal site. Apatite II can be used alone or in combination with clay, grout, or other components. This method removes metal from the leachate as it leaves the system.
Using the Apatite II as an additive to a generated waste steam or treatment within waste containers is a method in which Apatite II is added to the actual waste stream or within a container prior to disposal. Apatite II can be used alone or mixed with other constituents for mixed waste treatment. This method stabilizes the contaminant metal within the waste matrix before it is sent for disposal.
2.1.2 The Approach at Camp Stanley
The general application approach at CSSA is shown in Figure 2.1. Contaminated soils at SWMUs B‑8, B-20, B-24, B-28, and the Demo Dud area were excavated, ordnance pieces greater than three-quarters of an inch were removed, and soils were mounded into 18 piles of approximately 500 cubic yard each. The approach will be to treat a pile of lead-contaminated soil by mixing it with 5 percent Apatite II. The Apatite II mixed into the soil will provide both phosphate to solution to induce precipitation of the soluble lead into lead-pyromorphite, and nucleation sites upon which the lead-pyromorphite will precipitate. The lead in solution comes from the particulate lead, either as metal or as weathered oxide coatings. Therefore, during this treatment the lead particulate is isolated from the environment as it moves from the original contaminant phase to the new insoluble phase. A clean 2-foot soil or rock cover prevents human and animal intrusion, and a thin underlayer of Apatite II insures against unexpected fast-path flow, poor mixing, or other adverse boundary conditions.
Pending a decision by CSSA concerning the amount of soil to be treated and the final disposition of the treated soil, the anticipated field study is as follows:
Spread the treated soil over a developed site area, underlain by a thin layer of Apatite II, and underlain by a leachate-collection system;
Cover the treated soil with uncontaminated soil or rock to prevent erosion and intrusion; and
Stabilize the surface by mulching or vegetating with grasses or other applicable native vegetation.
The thickness of each layer will be determined during the field implementation based on practical and regulatory issues. However, it is anticipated that the underlying Apatite II layer will be 3 to 6 inches with 3 to 4 feet of treated soil, and 2 feet of clean soil with vegetated cover or 1 foot layer of rock/mulch material placed over the Apatite II layer.
To avoid potentially contaminating a clean area, treatment is expected to occur within the boundaries of an existing SWMU. The treatment site will be underlain by a liner and include a leachate collection system for subsequent monitoring. The site will be irrigated to test the long-term performance of the system. No further action is necessary. Monitoring will continue for four quarters under the ESTCP and beyond if necessary until regulatory agencies are satisfied that success has been achieved.
There are no key design criteria other than effectively mixing the soil and the Apatite II and emplacing it appropriately so it is stable from a slope-stability/soil-stability standpoint, e.g., it will not wash away in a flooding event.
Table 2.2 - Chronological Summary of Development of the Technology
| 1997-99 | Judith Wright doctoral dissertation work in which she first demonstrates the stability of metals in fossil apatite over geologic time scales. |
| 1997-01 | Judith Wright continues research on apatites and metals. Enlists the help of James Conca. |
| 1997-01 | Judith Wright obtains SERDP funding to investigate the use of apatites in remediating Pb. Performs work with James Conca at WSU. |
| 1994 | UFA Ventures, Inc. formed as a small business to investigate environmental problems and develop new technologies. Judith Wright and James Conca, principals. |
| 1996-98 | UFA Ventures Obtained USEPA SBIR funding to develop Apatite II for acid mine drainage. |
| 1999-00 | UFA Ventures obtained ESTCP funding. James Conca goes to LANL. Judith Wright is President of UFA Ventures. |
| 2000 | ESTCP Treatability Study for CSSA performed by UFA Ventures. |
| 2001 | ESTCP Field Demonstration scheduled for CSSA. |
2.2 - Previous Testing of the Technology
Previous work with lead, zinc, cadmium, uranium, and plutonium has shown performance of Apatite II under a variety of conditions. Under a SERDP project, UFA Ventures, Inc. (UFAV) investigated the metal-stabilization potential of reactive phosphates and other sorptive media in soil mixing and permeable reactive barrier situations at the Bunker Hill Mining District in northern Idaho. Soil at Bunker Hill was contaminated with lead, zinc, and cadmium up to 4,000 ppm, and groundwaters had concentrations of lead, zinc, cadmium, and copper up to 10 ppm, 250 ppm, 1 ppm and 20 ppm, respectively. Treatability studies using columns of soil mixed with various amounts of apatite showed that PIMS-treated soils did not leach any metal above the detection limits of ICP/MS (5 ppb for lead and cadmium, and 25 ppb for zinc). Even as little as 1 percent apatite by weight was effective. In permeable reactive barriers, Apatite II was orders of magnitude more effective than any other media, including bone char, iron filings, zeolites, CabSorb, C-Sorb, and activated charcoal (Wright et al., 1995; Chen et al., 1997; Conca, 1997; Conca, 1998). X-ray defraction showed well-crystallized lead-pyromorphite precipitated in both the PIMS-treated soil and in the barriers of apatite. Similar results were obtained for uranium during treatability studies of remediation of uranium-contaminated soils from a depleted-uranium firing range; the highly insoluble uranium-phosphate mineral, autunite, was precipitated on the Apatite II, and the soil was cleaned up to below the release criteria for placement back to the site. Apatite II was also successfully tested as a liner in treatability studies to prevent plutonium from leaching and escaping waste disposal drums.
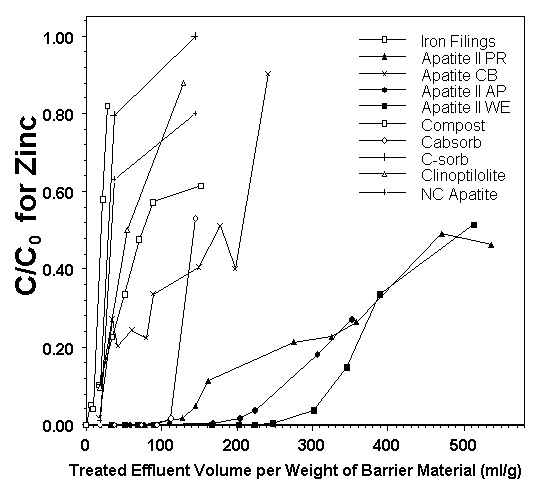
Under a USEPA SBIR grant to UFA Ventures, Inc., the efficacy of emplacement to a permeable reactive barrier to treat lead, cadmium, and zinc in seep waters from the Success Mine tailings pile was investigated. A feasibility study was performed on various materials, including different sources of apatite (three Apatite II formulations [Apatite WE, PR, and AP] and cow-bone apatite [Apatite CB]), iron-filings, compost/woodchip/gravel mixture, two zeolites, a polymer used in remediation of mine wastes, and activated charcoal. The Apatite II performed best with respect to all metals. Results for zinc are shown in Figure 2.2 which plots contaminant concentration in the effluent normalized to the influent C/C0, versus the volume of water passing through the column normalized to the weight of barrier material. Similar results occurred for cadmium (Conca, 1997).
UFA Ventures performed batch leach tests to determine the relative effect of Apatite II on the reduction of the bioavailability of lead from ingested contaminated soils in migratory water fowl. Contaminated soil was collected and treated with 1 percent, 10 percent, and 50 percent Apatite II by weight. These were run in batch tests adjusted to pH 2.4 with HCl. The substrate to water ratio was the standard 1:10 and mixtures were shaken for 24 hours. The solutions were then filtered through 0.2 micron filters and analyzed for lead and zinc. Results are given below. The presence of Apatite II, even at pH 2, dramatically decreased the amount of lead and zinc in solution. Toxicity tests on live water fowl also show significant decreases in lead uptake into organs and bone with the addition of Apatite II to contaminated soil (unpublished proprietary reports).
Table 2.3 - Results of Batch Leach Tests
| Soil | Lead (ppb) | Zinc (ppb) | pH |
| Untreated Soil | 24,000 | 2,800 | 4.28 |
| Soil with 1% Apatite II | 174 | 140 | 6.07 |
| Soil with 10% Apatite II | 104 | <50 | 6.87 |
| Soil with 50% Apatite II | 240 | <50 | 7.02 |
As a result of these studies, the State of Idaho proceeded with emplacement of an Apatite II permeable reactive barrier at the Success Mine site. Monitoring results from the field are not yet available.
Figure 2.1 - PIMS Soil Mixing for Pb Stabilization
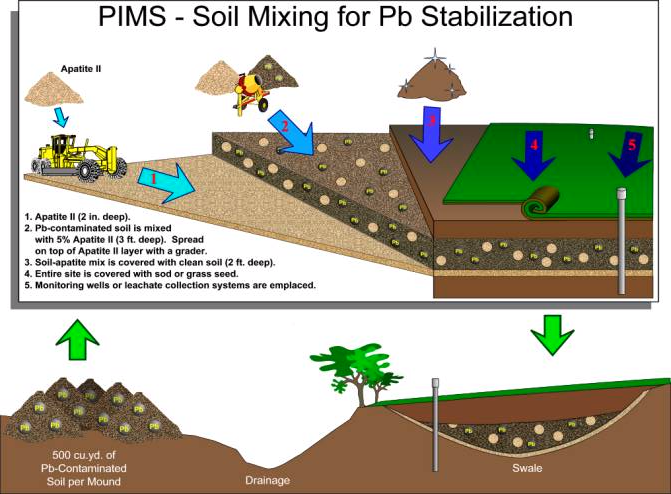
Figure 2.2 - Breakthrough of Dissolved Zinc through Various Permeable Reactive Barriers Using Contaminated Groundwater from the Success Mine Site. Apatite II Performed Best
2.3 - Factors Affecting Cost and Performance
Factors affecting cost of this treatment method include cost of the Apatite II (stable at about $575/ton) and normal construction costs of soil handling (e.g., mixing, handling, etc.) which are also well established and stable. Performance will be only slightly affected by how much Apatite II is used and how well it is mixed (all previous benchscale treatability studies purposely used gross mixing techniques to reflect field conditions). Climate will only slightly affect performance of the demonstration.
2.4 - Advantages and Limitations of the Technology
The DoD Complex has over 100 million cubic yards of metal contaminated soils. The baseline method for clean up of these soils is removal and disposal in a landfill after stabilization of the soil (normally with Portland cement) or some other applicable treatment. Cost of disposal varies from site to site, but can range from $50 to $150 per cubic yard, even if there is appropriate landfill space available. Unfortunately, only a fraction of the required landfill space is available for all of DoD's lead-contaminated soils, and landfill space will decrease as the liability for RCRA waste becomes more expensive. Although exact costs will be determined by this demonstration, preliminary cost estimates for treatment of contaminated soils with PIMS are estimated at less than $50 per cubic yard of contaminated soil. This results in a potential cost savings approaching $10 billion over the entire DoD Complex. Soil washing, phytoextraction, soil sieving and sluicing are alternative technologies, but the site study soils have both finely dispersed lead and pieces of lead from ordnance, making those alternatives difficult as each one treats only one or the other component. Costs of these alternatives range from approximately $100 to $500 per cubic yard of soil and depend strongly upon site conditions. For soil washing techniques, the wash solution, usually acidic for metals other than uranium, and the large buffering capacity of the soil at CSSA would render soil washing ineffective. Sieving removes the larger pieces of particulate lead however, the technique is ineffective at reducing the soluble lead from the finely dispersed lead phases. Pyhtoextraction involves growing genetically engineered plant to uptake soluble lead from soils. Phytoextraction technology is an innovative and promising technology, however, cost may be prohibitive in reaching cleanup goals at CSSA.
The limitation of this technology is due to the fact that lead is not removed from the system, but stabilized within the system. Therefore, for sites requiring removal of the contaminant to background concentrations for soil media, and not just removal from solution or groundwater, this technology may not be acceptable. The other limitation is the ability to mix Apatite II into the soil either before emplacement or in situ. Because the Apatite II mineral must come into contact with the metal species, adequate mixing is essential in ensuring sufficient reaction time. In some instances (e.g., gravel/cobbles) in situ and/or ex situ mixing may be difficult in providing the necessary contact with the Apatite II mineral, but it would be a rare instance that such a material would be contaminated. If it is not possible to mix Apatite II within the media, then this technology may not be acceptable.